Scientists receive Nobel Prize for metal-organic frameworks
The development of novel molecular constructions
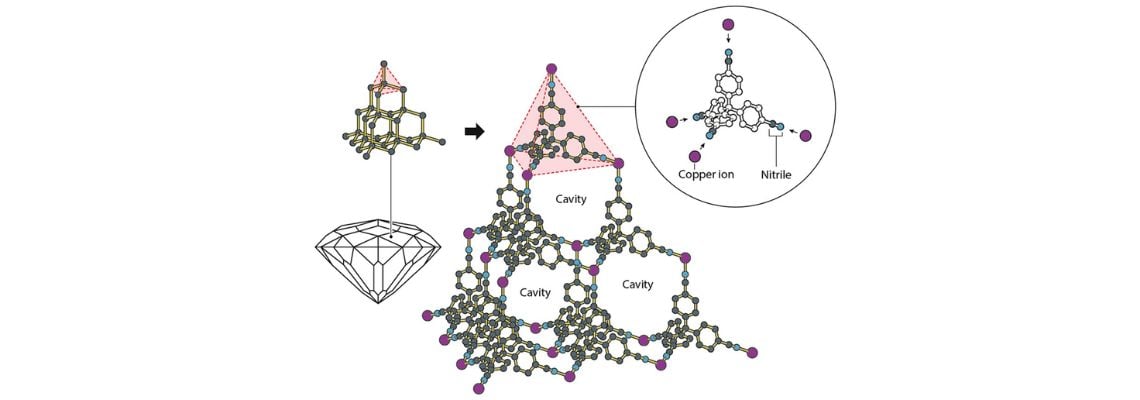
Richard Robson (University of Melbourne, Australia) began research into metal-organic frameworks (MOFs) in 1989. His research tested new ways of utilising the inherent properties of atoms, such as combining positively charged copper ions with a four-armed molecule; this had a chemical group that was attracted to copper ions at the end of each arm.
Although his molecular construction was unstable and prone to collapse, his combination had bonded to form a well-ordered, spacious crystal, shaped like a diamond but filled with innumerable cavities.
Stability was achieved through the efforts of Susumu Kitagawa (Kyoto University, Japan) and Omar M. Yaghi (University of California, Berkeley, USA), who worked separately between 1992 and 2003.
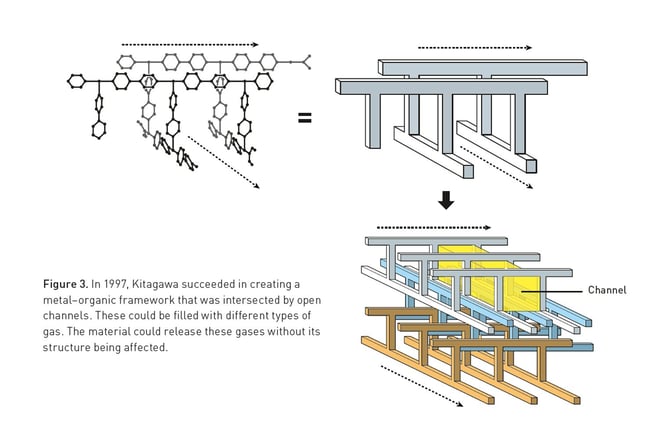
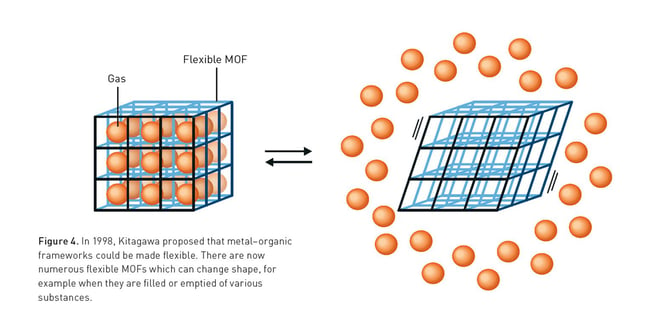
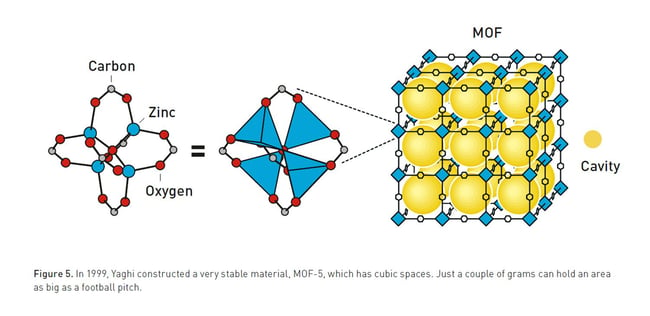
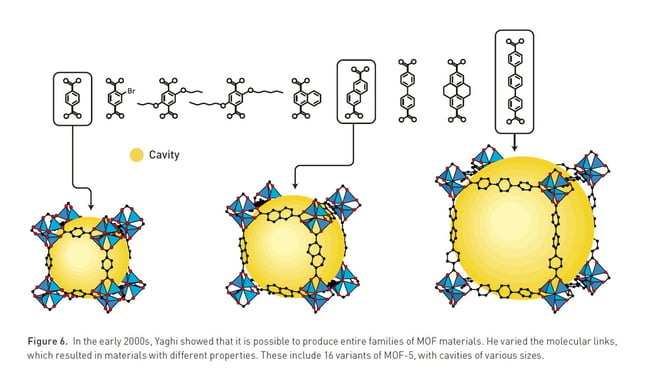
Kitagawa showed that gases can flow in and out of the constructions and predicted that MOFs could be made flexible. Yaghi created a very stable MOF and showed that it can be modified using rational design, giving it novel properties.
Robson, Kitagawa and Yaghi will share the prize of 11 million Swedish kroner (€1 million).
A bit like mobile cat litter
So why use MOFs rather than a widely available technology like zeolites?
Zeolites are microporous, crystalline materials, often described as molecular sieves. They are sold in large volumes - millions of tonnes each year - for use in a wide variety of industries, including water purification, and uses such as cat litter.
MOFs have the advantage of being more flexible than zeolites, which allows the structures to change shape when the cavities are filled – for example, by water. MOFs return to their original shape when the cavities are emptied.
Yaghi developed MOFs with high thermal stability and large storage cavities. While zeolites have relatively small internal capacity, a few grams of Yaghi’s MOFs have a surface area equivalent to a football field.
Yaghi, the keynote speaker at Aquatech Amsterdam 2025, developed MOFs with high thermal stability and large storage cavities. While zeolites have relatively small internal capacity, a few grams of Yoghi’s MOFs have a surface area equivalent to a football field.
What does this mean for the water sector?
Chemists can reorganise and vary the building blocks used in MOFs to change their function. For example, by adjusting the combination of metals and organic bridges of varying lengths, the pore size can be fine-tuned so that specific molecules fit inside the cavities. This allows chemists to design MOFS to target, capture and store specific substances, for example, per- and polyfluoroalkyl (PFAS) substances. In addition, they can also drive reactions and conduct electricity.
Awarding the prize, Heiner Linke, chair of the Nobel Committee for Chemistry, said: “Metal–organic frameworks have enormous potential, bringing previously unforeseen opportunities for custom-made materials with new functions.”
These functions include breaking down traces of pharmaceuticals in the environment and capturing carbon dioxide. More pertinent to the water industry, MOFs have the potential to be used for adsorption applications, such as separating PFAS and other pollutants from water and harvesting water directly from the air.
Atmospheric water harvesting
In 2023, utilising the huge internal surface area of Yaghi’s MOF, his team demonstrated the material’s potential for capturing water from the air in even the driest environments on earth – Death Valley – using only passive energy from the sun.
In a paper published in Chem, the authors of Metal-organic frameworks for water vapor adsorption suggest that MOFs could potentially open up certain atmospheric water harvesting technologies, for example, fog harvesting and dewing to parts of the world they are normally precluded from because alternative methods require high humidity and large energy consumption. MOFs overcome these limitations: water vapour condenses on the adsorbent MOF in ambient environmental temperatures and is released through application or pressure of heat (this could be from the sun, or from waste heat). The vapour is passed to a condenser, where it can be collected as fresh water.
Future uses for MOFs in water treatment
Research conducted by the Netherlands-based KWR Water Research Institute suggested the large internal surface area of MOFs compared to activated carbon could be used for PFAS remediation technology. The report also suggested MOFs can be used to target, capture and remove organic ions, such as phosphate, nitrate, ammonium, sulfide, and sulfate. It also suggests the removal of pollutants such as heavy metals and pharmaceutical residues, separation of different types of ions, and salt adsorption.
Future applications include use in purifying surface and groundwater streams and to increase pressure-driven membrane processes, such as reverse osmosis and nanofiltration.




